- Number Of Protons In An Atom Quizlet
- Number Of Protons In An Atom
- Number Of Protons In An Atom Of An Element
The number of protons in an atom is equal to its atomic number. In a neutral atom, the number of electrons is equal to that of protons. If the atom is negatively charged, add it to the number of. The nucleus is composed of protons (charge = +1; mass = 1.007 atomic mass units (μ) and neutrons. The number of protons in the nucleus is called the atomic number Z and defines which chemical element the nucleus represents. The number of neutrons in the nucleus is called the neutron number N, whereas the total number of neutrons and protons. Atomic Number: Number of protons and it is also the number of electrons in an atom of an element. Element’s Symbol: An abbreviation for the element. Elements Name Atomic Mass/Weight: Number of protons + neutrons. The number of protons, neutrons, and electrons in an atom can be determined from a set of simple rules. The number of protons in the nucleus of the atom is equal to the atomic number (Z).The number of electrons in a neutral atom is equal to the number of protons.; The mass number of the atom (M) is equal to the sum of the number of protons and neutrons in the nucleus.
Science
1. Which of the following best describes an atom? A) protons and electrons grouped together in a random pattern B) protons and electrons grouped together in an alternating pattern C) a core of protons and neutrons surrounded by
science
6. The table above shows the number of protons in an atom of four different elements. According to the periodic table, which of these elements is a metal? * A.T B.Q C.X D.Z Element Number Of Protons T 7 Q 15 X 12 Z 17
Periodic Table
1. The atomic mass of an element is A. the sum of the protons and neutrons in one atom of the element.** B. twice the number of protons in one atom of the element. C. a ration based on the mass of a carbon-12 atom D. a weighted
Science Elements
The table above shows the number of protons in an atom of four different elements. According to the periodic table, which of these elements is a metal? * A.T B.Q C.X D.Z Element/ Number Of Protons T/ 7 Q/ 15 X/ 12 Z/ 17 (I
You can view more similar questions or ask a new question.
How do you determine how many protons, neutrons, and electrons are in an atom?
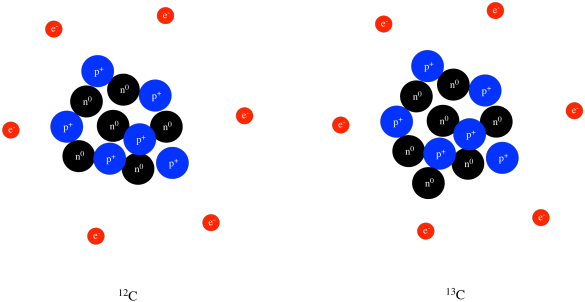
In order to determine the number of protons, neutrons and electrons that are in an atom, the first thing you need to do is find the atomic number and the atomic mass of the element you are interested in. You can use the Periodic Table of Elements to find this information. Let's use mercury as an example:
From the Table of Elements, we can see that mercury's atomic number is 80 and its atomic mass is 200.59. We'll need these numbers to get the information you want. The atomic number is the number of protons in an atom's nucleus, so we can tell right away that an atom of mercury contains 80 protons. Atoms, by definition, are electrically neutral. Protons carry a positive charge, so the nucleus of an atom of mercury carries a charge of +80. This positive charge is balanced by electrons, which carry a negative charge. 80 electrons are needed to balance the 80 protons. The atomic mass is sort of an average of all of the different isotopes of an element. Mercury's atomic mass is 200.59, but we can round that to 201 (this is known as the mass number). This is a tally of the total number of particles in an atom's nucleus, so an 'average' atom of mercury contains 201 particles in its nucleus. We know that 80 of those particles are protons. The rest must be neutrons. So, an atom of mercury contains 201 - 80 = 121 neutrons. In summary:
| protons = atomic number |
| electrons = atomic number |
| neutrons = rounded atomic mass - atomic number |
Number Of Protons In An Atom Quizlet
We have a game on our site that you can use to practice these calculations. You can find it at: http://education.jlab.org/elementmath/


Author:
Number Of Protons In An Atom
Steve Gagnon, Science Education Specialist (Other answers by Steve Gagnon)
Related Pages:
Number Of Protons In An Atom Of An Element
For questions about this page, please contact Steve Gagnon.
